
This page is all about polymer crystals. No, this page has nothing to do with polymers as used by the new age community. We"re talking about another kind of crystal here. The kind of crystal we"re talking about here is any object in which the molecules are arranged in a regular order and pattern. Ice is a crystal. In ice all the water molecules are arranged in a specific manner. So is table salt, sodium chloride. (Oddly, your mother"s good crystal drinking glasses are not crystal at all, as glass is an amorphous solid, that is, a solid in which the molecules have no order or arrangement.)
To understand all this talk of crystals and amorphous solids, it helps to go home. Go home? Why? So you can look in your sock drawer, that"s why. You see, some people are very neat and orderly. When they put their socks away they fold them and stack them very neatly. Like this:

Other people don"t really care about how neat their sock drawers look. Such folk will just throw their socks in the drawer in one big tangled mess. Their sock drawers look like this:

Polymers are just like socks in that sometimes they are arranged in a neat orderly manner, like the sock drawer in the top picture. When this is the case, we say the polymer is crystalline. Other times there is no order, and the polymer chains just form a big tangled mess, like the socks in the bottom picture. When this happens, we say the polymer is amorphous.
We"re going to talk about the neat and orderly crystalline polymers on this page.
So what kind of arrangements do the polymers like to form?
They like to line up all stretched out, kind of like a neat pile of new boards down at the lumber yard.
But they can"t always stretch out that straight. In fact, very few polymers can stretch out perfectly straight, and those are ultra-high molecular weight polyethylene, and aramids like Kevlar and Nomex. Most polymers can only stretch out for a short distance before they fold back on themselves. You can see this in the picture.

For polyethylene, the length the chains will stretch before they fold is about 100 angstroms.
But not only do polymers fold like this. Polymers also form stacks of these folded chains. There is a picture of a stack, called a lamella, right below.

Of course, it isn"t always as neat as this. Sometimes part of a chain is included in this crystal, and part of it isn"t. When this happens we get the kind of mess you see below. Our lamella is no longer neat and tidy, but sloppy, with chains hanging out of it everywhere!

Of course, being indecisive, the polymer chains will often decide they want to come back into the lamella after wandering around outside for awhile. When this happens, we get a picture like this:

This is the switchboard model of a polymer crystalline lamella. Because we like you, we"re going to tell you that when a polymer chain doesn"t wander around outside the crystal, but just folds right back in on itself, like we saw in the first pictures, that is called the adjacent re-entry model.
Amorphousness and Crystallinity
Are you wondering about something? If you look at those pictures up there, you can see that some of the polymer is crystalline, and some is not! Yes folks, most crystalline polymers are not entirely crystalline. The chains, or parts of chains, that aren"t in the crystals have no order to the arrangement of their chains. We fancy bigshot scientists say that they are in the amorphous state. So a crystalline polymer really has two components: the crystalline portion and the amorphous portion. The crystalline portion is in the lamellae, and the amorphous potion is outside the lamellae. If we look at a wide-angle picture of what a lamella looks like, we can see how the crystalline and amorphous portions are arranged.

As you can see, lamella grow like the spokes of a bicycle wheel from a central nucleus. (Sometimes we bigshot scientists like to call these spokes "lamellar fibrils".) The fibrils grow out in three dimensions, so they really look more like spheres than wheels. The whole assembly is called a spherulite. In a sample of a crystalline polymer weighing only a few grams, there are many billions of spherulites.
In between the crystalline lamellae, there are regions where there is no order to the arrangement of the polymer chains. These disordered regions are the amorphous regions we were talking about.
As you can also see in the picture, a single polymer chain may be partly in a crystalline lamella, and partly in the amorphous state. Some chains even start in one lamella, cross the amorphous region, and then join another lamella. These chains are called tie molecules.
So you see, no polymer is completely crystalline. If you"re making plastics, this is a good thing. Crystallinity makes a material strong, but it also makes it brittle. A completely crystalline polymer would be too brittle to be used as plastic. The amorphous regions give a polymer toughness, that is, the ability to bend without breaking.
But for making fibers, we like our polymers to be as crystalline as possible. This is because a fiber is really a long crystal. Want to know more? Then visit the Fiber Page!
Many polymers are a mix of amorphous and crystalline regions, but some are highly crystalline and some are highly amorphous. Here are some of the polymers that tend toward the extremes:
| Some Highly Crystalline Polymers: | Some Highly Amorphous Polymers: |
| Polypropylene | Poly(methyl methacrylate) |
| Syndiotactic polystyrene | Atactic polystyrene |
| Nylon | Polycarbonate |
| Kevlar and Nomex | Polyisoprene |
| Polyketones | Polybutadiene |
Why?
Crystallinity and polymer structure

As you can see on the lists above, there are two kinds of polystyrene. There is atactic polystyrene, and there is syndiotactic polystyrene. One is very crystalline, and one is very amorphous.
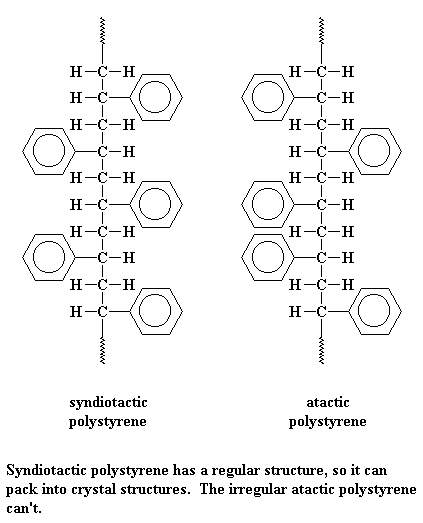
Syndiotactic polystyrene is very orderly, with the phenyl groups falling on alternating sides of the chain. This means it can pack very easily into crystals.
But atactic styrene has no such order. The phenyl groups come on any which side of the chain they please. With no order, the chains can"t pack very well. So atactic polystyrene is very amorphous.
Other atactic polymers like poly(methyl methacrylate) and poly(vinyl chloride) are also amorphous. And as you might expect, stereoregular polymers like isotactic polypropylene and polytetrafluoroethylene are highly crystalline.
Polyethylene is another good example. It can be crystalline or amorphous. Linear polyethylene is nearly 100% crystalline. But the branched stuff just can"t pack the way the linear stuff can, so it"s highly amorphous.

Crystallinity and intermolecular forces
Polyesters are another example. Let"s look at the polyester we call poly(ethylene terephthalate).

The polar ester groups make for strong crystals. In addition, the aromatic rings like to stack together in an orderly fashion, making the crystal even stronger.

How Much Crystallinity?
Remember we said that many polymers contain lots of crystalline material and lots of amorphous material. There"s a way we can find out how much of a polymer sample is amorphous and how much is crystalline. This method has its own page, and it"s called differential scanning calorimetry.
Keywords:
amorphous, crystal, first order transition
glass transition temperature, heat capacity, latent heat
second order transition, thermal transition
Note: Before you read this page, make sure you"ve read the glass transition page and the polymer crystallinity page.
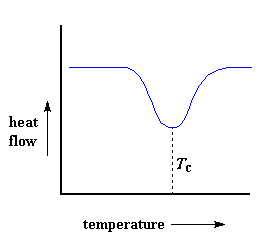
So how do we study what happens to a polymer when we heat it? The first step would be to heat it, obviously. And that"s what we do in differential scanning calorimetry, or DSC for short. We heat our polymer in a device that looks something like this: But more importantly, it makes sure that the two separate pans, with their two separate heaters, heat at the same rate as each other. Huh? Why wouldn"t they heat at the same rate? The simple reason is that the two pans are different. One has polymer in it, and one doesn"t. The polymer sample means there is extra material in the sample pan. Having extra material means that it will take more heat to keep the temperature of the sample pan increasing at the same rate as the reference pan. So the heater underneath the sample pan has to work harder than the heater underneath the reference pan. It has to put out more heat. By measuring just how much more heat it has to put out is what we measure in a DSC experiment. Specifically what we do is this: We make a plot as the temperature increases. On the x-axis we plot the temperature. On the y-axis we plot difference in heat output of the two heaters at a given temperature. The heat flow at a given temperature can tell us something. The heat flow is going to be shown in units of heat, q supplied per unit time, t. The heating rate is temperature increase T per unit time, t. Got it? Let"s say now that we divide the heat flow q/t by the heating rate T/t. We end up with heat supplied, divided by the temperature increase. Remember from the glass transition page that when you put a certain amount of heat into something, its temperature will go up by a certain amount, and the amount of heat it takes to get a certain temperature increase is called the heat capacity, or Cp. We get the heat capacity by dividing the heat supplied by the resulting temperature increase. And that"s just what we"ve done in that equation up there. We"ve figured up the heat capacity from the DSC plot. This means we"re now getting more heat flow. This means we"ve also got an increase in the heat capacity of our polymer. This happens because the polymer has just gone through the glass transition. And as you learned on the glass transition page, polymers have a higher heat capacity above the glass transition temperature than they do below it. Because of this change in heat capacity that occurs at the glass transition, we can use DSC to measure a polymer"s glass transition temperature. You may notice that the change doesn"t occur suddenly, but takes place over a temperature range. This makes picking one discreet Tg kind of tricky, but we usually just take the middle of the incline to be the Tg. When polymers fall into these crystalline arrangements, they give off heat. When this heat is dumped out, it makes the little computer-controlled heater under the sample pan really happy. It"s happy because it doesn"t have to put out much heat to keep the temperature of the sample pan rising. You can see this drop in the heat flow as a big dip in the plot of heat flow versus temperature: This dip tells us a lot of things. The temperature at the lowest point of the dip is usually considered to be the polymer"s crystallization temperature, or Tc. Also, we can measure the area of the dip, and that will tell us the latent energy of crystallization for the polymer. But most importantly, this dip tells us that the polymer can in fact crystallize. If you analyzed a 100% amorphous polymer, like atactic polystyrene, you wouldn"t get one of these dips, because such materials don"t crystallize. Also, because the polymer gives off heat when it crystallizes, we call crystallization an exothermic transition. Remember that heat that the polymer gave off when it crystallized? Well when we reach the Tm, it"s payback time. There is a latent heat of melting as well as a latent heat of crystallization. When the polymer crystals melt, they must absorb heat in order to do so. Remember melting is a first order transition. This means that when you reach the melting temperature, the polymer"s temperature won"t rise until all the crystals have melted. This means that the little heater under the sample pan is going to have to put a lot of heat into the polymer in order to both melt the crystals and keep the temperature rising at the same rate as that of the reference pan. This extra heat flow during melting shows up as a big peak on our DSC plot, like this: We can measure the latent heat of melting by measuring the area of this peak. And of course, we usually take the temperature at the top of the peak to be the polymer"s melting temperature, Tm. Because we have to add energy to the polymer to make it melt, we call melting an endothermic transition. If you look at the DSC plot you can see a big difference between the glass transition and the other two thermal transitions, crystallization and melting. For the glass transition, there is no dip, and there"s no peak, either. This is because there is no latent heat given off, or absorbed, by the polymer during the glass transition. Both melting and crystallization involve giving off or absorbing heat. The only thing we do see at the glass transition temperature is a change in the heat capacity of the polymer. Because there is a change in heat capacity, but there is no latent heat involved with the glass transition, we call the glass transition a second order transition. Transitions like melting and crystallization, which do have latent heats, are called first order transitions. The first thing we have to do is measure the area of that big peak we have for the melting of the polymer. Now our plot is a plot of heat flow per gram of material, versus temperature. Heat flow is heat given off per second, so the area of the peak is given is units of heat x temperature x time-1 x mass-1. We usually would put this in units such as joules x kelvins x (seconds)-1 x (grams)-1: Now we"re going to subtract the two: Now with our magic number H" we can figure up the percent crystallinity. We"re going to divide it by the specific heat of melting, Hc*. The specific heat of melting? That"s the amount of heat given off by a certain amount, usually one gram, of a polymer. H" is in joules, and the specific heat of melting is usually given in joules per gram, so we"re going to get an answer in grams, which we"ll call mc.
Differential scanning calorimetry is a technique we use to study what happens to polymers when they"re heated. We use it to study what we call the thermal transitions of a polymer. And what are thermal transitions? They"re the changes that take place in a polymer when you heat it. The melting of a crystalline polymer is one example. The glass transition is also a thermal transition.
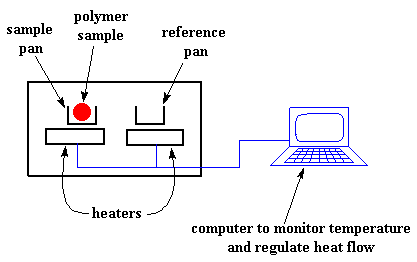 It"s pretty simple, really. There are two pans. In one pan, the sample pan, you put your polymer sample. The other one is the reference pan. You leave it empty. Each pan sits on top of a heater. Then you tell the nifty computer to turn on the heaters. So the computer turns on the heaters, and tells it to heat the two pans at a specific rate, usually something like 10 oC per minute. The computer makes absolutely sure that the heating the rate stays exactly the same throughout the experiment.
It"s pretty simple, really. There are two pans. In one pan, the sample pan, you put your polymer sample. The other one is the reference pan. You leave it empty. Each pan sits on top of a heater. Then you tell the nifty computer to turn on the heaters. So the computer turns on the heaters, and tells it to heat the two pans at a specific rate, usually something like 10 oC per minute. The computer makes absolutely sure that the heating the rate stays exactly the same throughout the experiment.
Heat Capacity
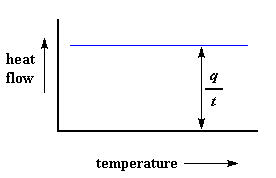
We can learn a lot from this plot. Let"s imagine we"re heating a polymer. When we start heating our two pans, the computer will plot the difference in heat output of the two heaters against temperature. That is to say, we"re plotting the heat absorbed by the polymer against temperature. The plot will look something like this at first.
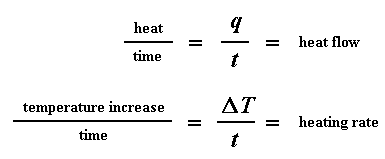
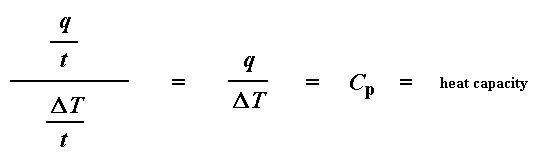
The Glass Transition Temperature
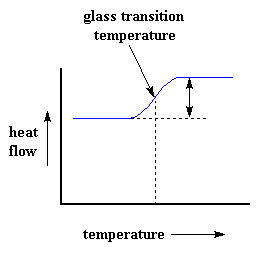
Of course, we can learn a lot more than just a polymer"s heat capacity with DSC. Let"s see what happens when we heat the polymer a little more. After a certain temperature, our plot will shift upward suddenly, like this:
Crystallization
But wait there is more, so much more. Above the glass transition, the polymers have a lot of mobility. They wiggle and squirm, and never stay in one position for very long. They"re kind of like passengers trying to get comfortable in airline seats, and never quite succeeding, because they can move around more. When they reach the right temperature, they will have gained enough energy to move into very ordered arrangements, which we call crystals, of course.
Melting
Heat may allow crystals to form in a polymer, but too much of it can be their undoing. If we keep heating our polymer past its Tc, eventually we"ll reach another thermal transition, one called melting. When we reach the polymer"s melting temperature, or Tm, those polymer crystals begin to fall apart, that is they melt. The chains come out of their ordered arrangements, and begin to move around freely. And in case you were wondering, we can spot this happening on a DSC plot.
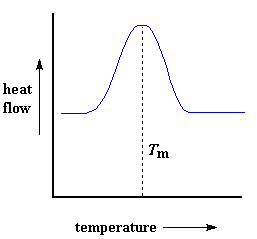
Putting It All Together
So let"s review now: we saw a step in the plot when the polymer was heated past its glass transition temperature. Then we saw a big dip when the polymer reached its crystallization temperature. Then finally we saw a big peak when the polymer reached its melting temperature. To put them all together, a whole plot will often look something like this:
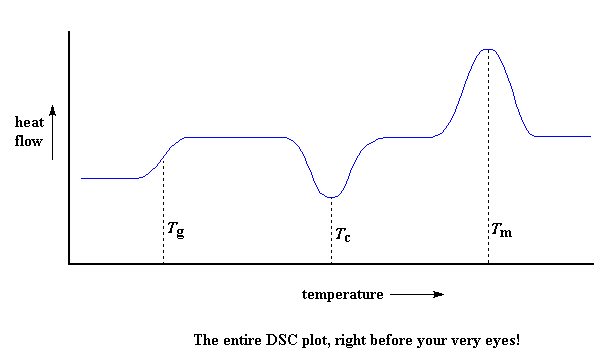
Of course, not everything you see here will be on every DSC plot. The crystallization dip and the melting peak will only show up for polymers that can form crystals. Completely amorphous polymers won"t show any crystallization, or any melting either. But polymers with both crystalline and amorphous domains, will show all the features you see above.
How much crystallinity?
DSC can also tell us how much of a polymer is crystalline and how much is amorphous. If you read the page dealing with polymer crystallinity, you know that many polymers contain both amorphous and crystalline material. But how much of each? DSC can tell us. If we know the latent heat of melting, ΔHm, we can figure out the answer.
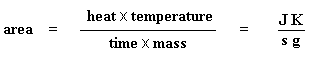 Got that? Don"t worry. It gets simpler. We usually divide the area by the heating rate of our dsc experiment. The heating rate is in units of K/s. So the expression becomes simpler:
Got that? Don"t worry. It gets simpler. We usually divide the area by the heating rate of our dsc experiment. The heating rate is in units of K/s. So the expression becomes simpler: 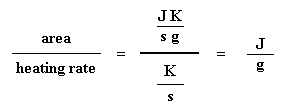 Now we have a number of joules per gram. But because we know the mass of the sample, we can make it simpler. We just multiply this by the mass of the sample:
Now we have a number of joules per gram. But because we know the mass of the sample, we can make it simpler. We just multiply this by the mass of the sample: 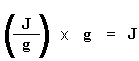 Now we just calculated the total heat given off when the polymer melted. Neat, huh? Now if we do the same calculation for our dip that we got on the DSC plot for the crystallization of the polymer, we can get the total heat absorbed during the crystallization. We"ll call the heat total heat given off during melting Hm, total, and we"ll call the heat of the crystallization Hc, total.
Now we just calculated the total heat given off when the polymer melted. Neat, huh? Now if we do the same calculation for our dip that we got on the DSC plot for the crystallization of the polymer, we can get the total heat absorbed during the crystallization. We"ll call the heat total heat given off during melting Hm, total, and we"ll call the heat of the crystallization Hc, total.
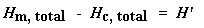 Why did we just do that? And what does that number H" mean? H" is the heat given off by that part of the polymer sample which was already in the crystalline state before we heated the polymer above the Tc. We want to know how much of the polymer was crystalline before we induced more of it to become crystalline. That"s why we subtract the heat given off at crystallization. Is everyone following me?
Why did we just do that? And what does that number H" mean? H" is the heat given off by that part of the polymer sample which was already in the crystalline state before we heated the polymer above the Tc. We want to know how much of the polymer was crystalline before we induced more of it to become crystalline. That"s why we subtract the heat given off at crystallization. Is everyone following me?
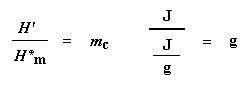 This is the total amount of grams of polymer that were crystalline below the Tc. Now if we divide this number by the weight of our sample, mtotal, we get the fraction of the sample that was crystalline, and then of course, the percent crystallinity:
This is the total amount of grams of polymer that were crystalline below the Tc. Now if we divide this number by the weight of our sample, mtotal, we get the fraction of the sample that was crystalline, and then of course, the percent crystallinity: 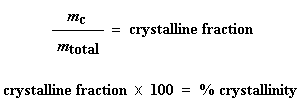 And that"s how we use DSC to get percent crystallinity
And that"s how we use DSC to get percent crystallinity





